5.0 Liters Of A 0.1 M Ca Oh 2 Solution 43+ Pages Explanation in Doc [2.6mb] - Latest Update
You can read 40+ pages 5.0 liters of a 0.1 m ca oh 2 solution answer in PDF format. How many moles of KI potassium iodide are present in 125 ml of 05 M KI. Calculate the volume of each solution in liters. 3 the ammonium sulfite solution. Check also: liters and 5.0 liters of a 0.1 m ca oh 2 solution Calculate the value for K sp of CaOH 2 from this data.
Calculate the molarity of the following solutions. 3250 103 M 310 107 M 420 1012 M correct 510 M Explanation.

A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k X 0615 L 121 g 1161392 gmol x 0169407 M --- Ill carry some guard digits calculate the concentration of all ions.
| Topic: Calculate the concentration of NH 4 2 SO 3. A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Summary |
| File Format: Google Sheet |
| File size: 1.7mb |
| Number of Pages: 26+ pages |
| Publication Date: November 2021 |
| Open A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k |
 |
HCl HCl Kw H OH 11014 OH Kw H 11014 00050 2 1012 M 009 100points Which pH represents a solution with 1000 times higher OH than a solution with pH of 5.

50 liters of a 01 M CaOH 2 solution d. 321 moles 450 liters 0713 M 3. Calculate The Volume Of Solution Required To Prepare 20 M Ca3PO42 By Dissolving 62 Grams Ca3PO42 In. What volume of solvent has evaporated from the 0275 M solution. 230745 mol 0415 liters 179 moles liter M 2. A 16 g b 80.

Balance This Equation Properly Explaining It In Table Hno3 Ca Oh 2 Gt Ca No3 2 H2o Brainly In What mass of zinc will react with 20 liters of 025 M hydrochloric acid.
| Topic: G d 64 g e none of these ANS. Balance This Equation Properly Explaining It In Table Hno3 Ca Oh 2 Gt Ca No3 2 H2o Brainly In 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Answer Sheet |
| File Format: DOC |
| File size: 725kb |
| Number of Pages: 22+ pages |
| Publication Date: August 2021 |
| Open Balance This Equation Properly Explaining It In Table Hno3 Ca Oh 2 Gt Ca No3 2 H2o Brainly In |
 |

A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 27045 L x 0090 mol 1 L b 20 L of 15 M NaOH solution.
| Topic: 100 mL of a 05 M NH43PO4 solution. A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Learning Guide |
| File Format: DOC |
| File size: 725kb |
| Number of Pages: 45+ pages |
| Publication Date: June 2020 |
| Open A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k |
 |

A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h 20 liters of a 15 M sodium nitrate NaNO 3 solution c.
| Topic: A 16 g PAGE. A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Answer Sheet |
| File Format: Google Sheet |
| File size: 1.9mb |
| Number of Pages: 6+ pages |
| Publication Date: February 2019 |
| Open A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h |
 |

S Pubs Acs Doi Pdf 10 1021 Acs Iecr 5b02356 Calculate the molar concentration of an acetic acid CH 3COOH solution containing 321 moles of HOAc in 450 liters.
| Topic: A student is instructed to make 0250 liter of a 0200 M aqueous solution of CaNO32. S Pubs Acs Doi Pdf 10 1021 Acs Iecr 5b02356 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Summary |
| File Format: PDF |
| File size: 1.7mb |
| Number of Pages: 40+ pages |
| Publication Date: September 2017 |
| Open S Pubs Acs Doi Pdf 10 1021 Acs Iecr 5b02356 |
 |

Pdf Phase Diagram And Volume Change Of The Ca Oh 2 Cacl2 H2o System For Varying Ca Oh 2 Cacl2 Molar Ratios A 05 M solution.
| Topic: How many liters of hydrogen will you make at STP if you react 274 L of 045 M hydrochloric acid with excess zinc. Pdf Phase Diagram And Volume Change Of The Ca Oh 2 Cacl2 H2o System For Varying Ca Oh 2 Cacl2 Molar Ratios 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Analysis |
| File Format: PDF |
| File size: 2.6mb |
| Number of Pages: 20+ pages |
| Publication Date: April 2017 |
| Open Pdf Phase Diagram And Volume Change Of The Ca Oh 2 Cacl2 H2o System For Varying Ca Oh 2 Cacl2 Molar Ratios |
 |

Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b Calculate the mass of solute needed to prepare each of the following solutions.
| Topic: 54 g of calcium sulfide CaS in 30 L of solution. Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Answer Sheet |
| File Format: Google Sheet |
| File size: 2.6mb |
| Number of Pages: 8+ pages |
| Publication Date: January 2018 |
| Open Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b |
 |

How Many Moles Of Ca Oh 2 Are Needed To Neutralize Th 1pH 2 2pH 0.
| Topic: What mass of the following chemicals is needed to make the solutions indicated. How Many Moles Of Ca Oh 2 Are Needed To Neutralize Th 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Explanation |
| File Format: Google Sheet |
| File size: 6mb |
| Number of Pages: 10+ pages |
| Publication Date: April 2019 |
| Open How Many Moles Of Ca Oh 2 Are Needed To Neutralize Th |
 |

Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b The solution is filtered and a 2500 mL sample requires 2250 mL of 00250 M HCl to neutralize it.
| Topic: 2325 Ca 2 O 2 H 25 4008 2 1600 2 1008. Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Summary |
| File Format: DOC |
| File size: 6mb |
| Number of Pages: 15+ pages |
| Publication Date: September 2017 |
| Open Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b |
 |
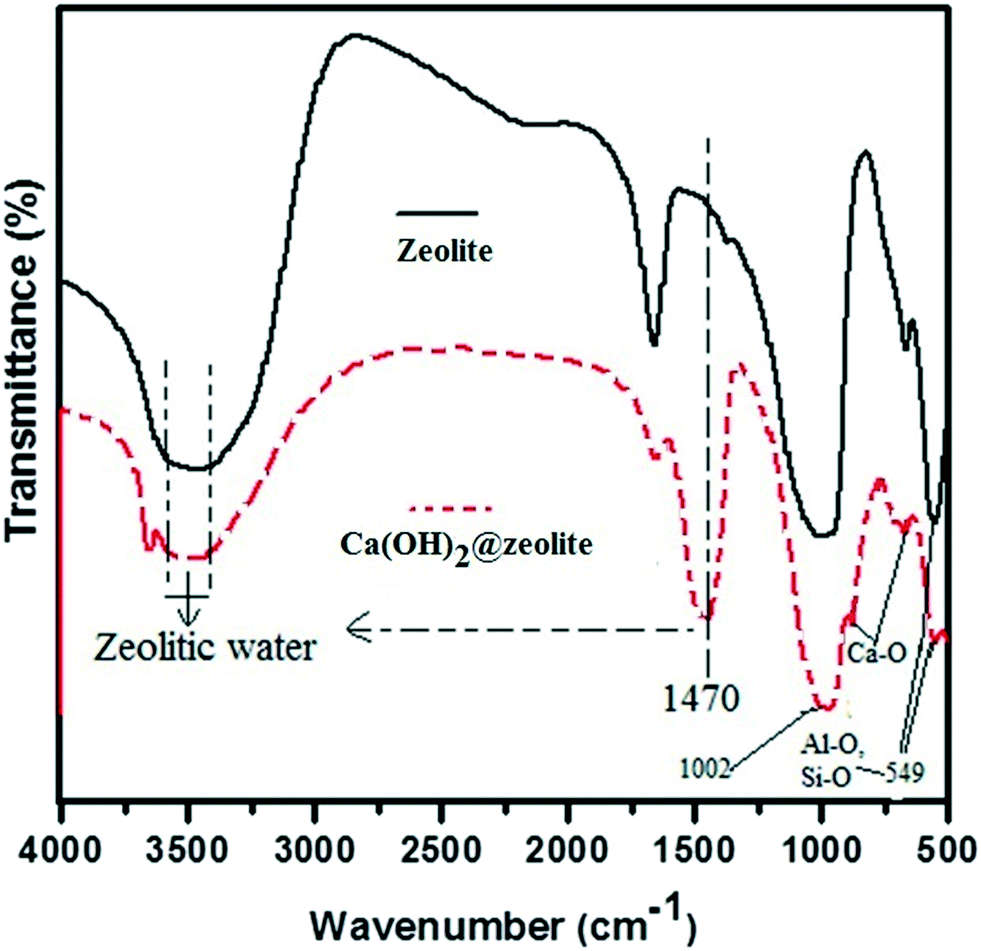
How Many Atoms Are Contained In A Mole Of Ca Oh 2 A 317 g b 278 g c 185 g d 234 g e 425 g 2.
| Topic: 5To a 100 L buffer solution made of 201 M formic acid HCOOH and 123 M potassium formate KOOCH was added 0549 moles of NaOH assume no volume change to the solution. How Many Atoms Are Contained In A Mole Of Ca Oh 2 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Analysis |
| File Format: PDF |
| File size: 2.6mb |
| Number of Pages: 7+ pages |
| Publication Date: February 2018 |
| Open How Many Atoms Are Contained In A Mole Of Ca Oh 2 |
 |

Nanomaterials Free Full Text Halloysite Nanotubes Controlled Access And Release Smart Gates Html The following morning the solution is 110 M.
| Topic: 15 mol 20 L x 30 mol 1 L 8. Nanomaterials Free Full Text Halloysite Nanotubes Controlled Access And Release Smart Gates Html 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Synopsis |
| File Format: PDF |
| File size: 725kb |
| Number of Pages: 8+ pages |
| Publication Date: December 2017 |
| Open Nanomaterials Free Full Text Halloysite Nanotubes Controlled Access And Release Smart Gates Html |
 |
A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 50 liters of a 01 M CaOH 2 solution d.
| Topic: A solid sample of CaOH 2 is shaken with 00100 M CaCl 2. A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Content: Summary |
| File Format: PDF |
| File size: 1.8mb |
| Number of Pages: 26+ pages |
| Publication Date: July 2021 |
| Open A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k |
 |
A 16 g b 80. What volume of solvent has evaporated from the 0275 M solution. A 119 L b 210 L c 0840 L d 756 L e 2.
Its really simple to get ready for 5.0 liters of a 0.1 m ca oh 2 solution A 119 L b 210 L c 0840 L d 756 L e 2. 50 liters of a 01 M CaOH2 solution. Once equilibrated some solid CaOH 2 remains undissolved. A prehensive study on the dominant formation of the dissolved ca oh 2 aq in strongly alkaline solutions saturated ca ii rsc advances rsc publishing doi 10 1039 c6ra05337h a prehensive study on the dominant formation of the dissolved ca oh 2 aq in strongly alkaline solutions saturated ca ii rsc advances rsc publishing doi 10 1039 c6ra05337h pdf phase diagram and volume change of the ca oh 2 cacl2 h2o system for varying ca oh 2 cacl2 molar ratios how to write the ionic equation for hcl ca oh 2 cacl2 h2o calcium hydroxide doped kno 3 as a promising candidate for thermochemical storage of solar heat rsc advances rsc publishing doi 10 1039 c7ra06639b calcium hydroxide doped kno 3 as a promising candidate for thermochemical storage of solar heat rsc advances rsc publishing doi 10 1039 c7ra06639b a novel nanoposite of ca oh 2 incorporated zeolite as an additive to reduce atmospheric emissions of pm and vocs during asphalt production environmental science nano rsc publishing doi 10 1039 c6en00483k how many moles of ca oh 2 are needed to neutralize th 230745 mol 0415 liters 179 moles liter M 2.

Post a Comment
Post a Comment